
PROCESSES
PRODUCTION OF ETHYLENE OXIDE
>


BACKGROUND
Ethylene oxide, also known as Oxirane is a colourless and flammable gas which liquefies when cooled below 12 degree celcius. It has a sweet and etheric odour, and is highly soluble in water and in organic solvents. Due to its single bond, it is a very reactive chemical. A versatile building block of chemistry, ethylene oxide and its derivatives are used in the production of products that we use everyday. Ethylene oxide has a minor but significant use in the sterilization of foods and medical supplies. Its derivatives are also used as ingredients in cleaners, cosmetics, shampoos, plasticizers, ointments, and pharmaceuticals. Moreover, ethylene oxide produced is used as an intermediate in the production of other useful chemicals such as ethylene glycols, ethanolamines and alcohol ethoxylates.
PROCESS DESCRIPTION
PROCESS FLOW OF ETHYLENE OXIDE
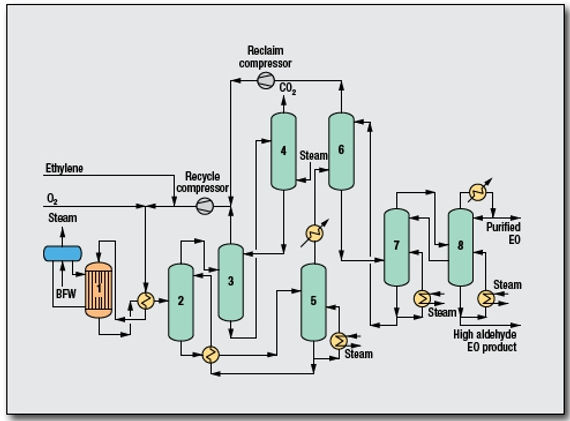
Steps of oxygen-based ethylene oxide processing
-
Ethylene and oxygen are mixed with recycled gas and fed to a multi-tubular reactor and react with silver catalyst at temperature of 200 to 300 Celsius and pressure of 10 to 20 bar. This reaction produced ethylene oxide, carbon dioxide, water and heat. The reaction heat which is generated from the highly exothermic reaction is used to generate steam.
-
Effluent gases from the reactor which mainly consist of ethylene oxide and carbon dioxide are first cooled and passed through a primary scrubber where the ethylene oxide is scrubbed from the gas by water as a dilute aqueous solution. Ethylene oxide containing water is then concentrated by stripping to recover it from the solution to produce crude ethylene oxide.
-
The crude ethylene oxide is fed to two series of fractionator columns to purify the ethylene oxide.
-
The gas leaving the primary scrubber is fed to the carbon dioxide removal section. The gas is first enter secondary scrubber where carbon dioxide is scrubbed by potassium carbonate and unabsorbed gas is recycled back to the reactor. The potassium carbonate containing carbon dioxide then enter desober where carbon dioxide is separated from the potassium carbonate and released to the atmosphere. The separated potassium carbonate is recycled back to the secondary scrubber.
REACTION CONDITIONS AND MECHANISM
Operating parameters used in the oxygen-based production of ethylene oxide

200 - 300 degree Celcius

1 s

Silver catalyst

70 - 75%

Exothermic
Reaction Mechanism

Share the amazing things customers are saying about your business. Double click, or click Edit Text to make it yours.

OUR ETHYLENE OXIDE BASED PRODUCTS


