
PROCESSES
PRODUCTION OF ETHYLENE
>
CRUDE OIL FRACTIONAL DISTILLATION
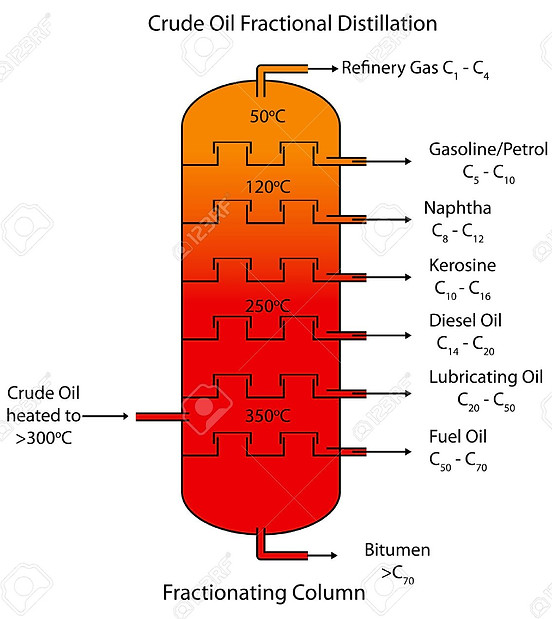
The oil refining process starts with a fractional distillation column. The various components of crude oil have different sizes, weights and boiling temperatures; so, the first step is to separate these components. Since the components of crude oils have different boiling temperatures, they can be separated easily by a process called fractional distillation. After going through the fractional distillation, crude oil is chemically processed to change one fraction into another. Finally, distillates and chemically processed fractions are treated to remove impurities.
The mechanisms for fractional distillation are as follow:-
-
Heating the mixture of the substances of crude oil (liquids) with different boiling points to a high temperature. Heating is usually done with high pressure steam to temperatures of about 600oC.
-
The mixture boils, forming vapor (gases). The vapor enters the bottom of a fractional distillation column that is filled with trays. The trays have many holes or bubble caps in them to allow the vapor to pass through.They increase the contact time between the vapor and the liquids in the column and help to collect liquids that form at various heights in the column. There is a temperature difference across the column (hot at the bottom, cool at the top).
-
The vapor rises in the column. As the vapor rises through the trays in the column, it cools.
-
When the substance in the vapor reaches a height where the temperature of the column is equal to the substance’s boiling point. It will condense to form a liquid. Substance with lowest boiling point will condense at the highest point in the column; substances with higher boiling points will condense lower in the column.
-
The trays collect the various liquid fractions. The collected liquid fractions may pass to condensers, which cool them further, and then go to storage tanks, or they may go to other areas for further chemical processing.
STEAM CRACKING OF NAPHTHA
Reactant
Naphtha
Reaction
Conditions
Endothermic

Operating Temperature
750-875oC
Operating Pressure
13 bar
Pyrolysis furnace
Pyrolysis is the thermal cracking of petroleum hydrocarboons with steam. The feedstocks (naphtha or natural gas) is cracked into ethylene and othe various products in a furnace. the feed stream is preheated by heat exchanger and mixed with steam and further heated to its incipient crackign temperature (500-680oC). the stream enters reactor and heated to cracking temperature (750-875oC). during this reaction, hydrocarbons in the feed are cracked into smaller molecules, producing ethylene and co-products
Chemical Reactions

Quality control variables:
-
Cracking coilsare designed to optimized the temperature and pressure profiles
-
steam helps to redue coking deposits by reacting with coke to form carbon dioxide, carbon monoxide and hydrogen and also reduces the catalytic effect of the reactor coil's metals.
-
decoking : burning out the coke with a mixture of steam and air to furnace efficiency.
QUENCHING SECTION

Gasoline Fractionator
Heavy fuel oils cuts are separated from the bulk of the effluent stream in the gasoline fractionator by direct contact with circulating pyrolysis oil. Function is to make a sharp separation between the heavy oil fraction from the gasoline and lighter fractions. The gasoline fractionator is only used in case of a liquid feedstock (naphtha).
Quench Tower
Further cooling is performed in the quench tower by circulating waters streams to minimize any further cracking. The quench tower operates as a partial condenser for the fractionator, condensing practically all the steam and most of the pyrolysis gasoline components.
COMPRESSION SECTION

Compression Train
The gas from quench tower is then compressed in a 4 or 5 stage compressor train to an optimum pressure for separating it into various components. Water and hydrocarbons are separated between stages and recycled. Acid gases are removed after the 3rd or 4th compression stage by scrubbing them with dilute caustic soda solution.
Refrigeration Train
The pyrolysis gas is the partially condensed over the stage of a refrigetaion system to about -165oC, where the hydrogen remains in the vapor stage. The stage condensates are fed to the demethanizer while hydrogen is withdrawn from the lowest temperature separator.
SEPARATION SECTION

Demethanizer
The Demethanizer is designed for complete separation of methane from ethylene and heavier components. The demethanizer overhead consists of methane with some impurities of hydrogen, CO and traces of ethylene. The demethanizer bottoms, consists of ethylene and heavier component, are sent to deethanizer.
Deethanizer
The dethanizer produces C2 hydrocarbons as overhead (acetylene, ethane and ethylene) and C¬3 and heavier hydrocarbons as bottom.
Acethylene Hydrogenation
The deethanizer overhead is heated and hydrogen is added to convert acetylene to ethylene and ethane (hydrogenation). The effluent contains less than 1 ppm of acetylene and traces of methane and hydrogen.
Ethylene Fractionator (C2 Splitter)
After acetylene removal, the dried gas enters an ethylene-ethane separator. Ethylene product is gain here while ethane being recycle.
